Abstract
Background Peripheral T-cell lymphomas (PTCL) are a rare and heterogeneous group of non-Hodgkin lymphomas of mature T-cell origin. Systemic PTCL (sPTCL) has mostly poor outcomes with conventional (CHOP-like) chemotherapy. Biomarker-driven, subtype-specific and personalized treatments are promising but unmet needs. Few benchmark studies exist to delineate the contemporary landscape of clinical and pathologic practice in the real-world setting to guide therapeutic development. We report herein the patterns of care and outcomes for PTCL patients enrolled in the LEO-MER multi-center prospective cohort study.
Methods Patients ≥ 18 years with newly diagnosed PTCL were prospectively enrolled in the University of Iowa/Mayo Clinic MER cohort (2002-2015) and the expanded 8 US center LEO cohort (2015-2020). Clinical, pathology, treatment, and outcome data were abstracted from medical records using a standard protocol. Pathology underwent expert re-review based on WHO criteria, with harmonization between the 2008 and 2016 classifications where appropriate. Overall survival (OS) was calculated from date of diagnosis to date of death or last follow-up. Event-free survival (EFS) was calculated from date of diagnosis to disease progression, initiation of 2nd line therapy, or death from any cause. EFS and OS were evaluated using Cox Model and Kaplan-Meier curves.
Results 1132 PTCL were identified (462 MER and 670 LEO), including 718 sPTCL (281 MER and 437 LEO). sPTCL subtypes included PTCL-NOS (N=252, 35.1%), anaplastic large cell lymphoma (ALCL; N=180, 25.7% [74 ALK+, 106 ALK-]), angioimmunoblastic T-cell lymphoma (AITL, N=162, 22.6%), extranodal NK-TCL (N=50, 7.0%), adult T-cell leukemia/lymphoma (N=32, 4.5%), enteropathy-associated TCL (N=19, 2.6%), and hepatosplenic TCL (N=13, 1.8%). Median age at diagnosis was 60 years. M:F ratio was 1.5. Comparing LEO to MER, nonwhite population was 23.8% vs 8.5%, and Hispanic was 10.1% vs 2.2%. Clinical characteristics were comparable between MER and LEO overall: PS≥2, 21.2%; elevated LDH, 50.4%; stage III-IV, 67.5%; and IPI 2-5, 61.6%.
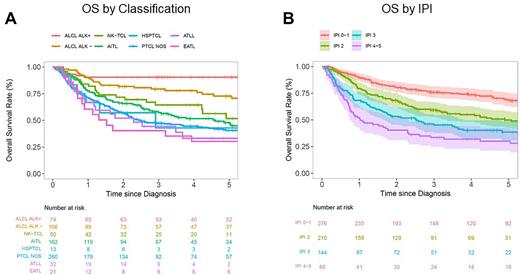
The most common 1st line chemotherapy regimens were anthracycline-based (N=514, 72.2%), of which 205 (39.9%) included etoposide (50 [24.4%] in MER, 155 [50.2%] in LEO) and 43 (8.4%) included brentuximab vedotin (0 in MER and 43 [13.9%] in LEO). 73 patients (10.3%) underwent consolidative SCT following induction chemotherapy (70 auto and 3 allo), and 64 patients (11.1%) received frontline therapy on clinical trials. At a median follow-up of 9.3 years (95% CI: 8.9-10.8) in MER and 3.2 years (95% CI: 3.1-3.6) in LEO, 177 (MER) and 250 (LEO) events, and 150 (MER) and 183 (LEO) deaths were observed, respectively. The 2-year EFS and 3-year OS estimates for sPTCL were 51.6% (95% CI: 46.0%-57.8%) and 61.3% (95% CI: 55.9%-67.3%) in MER, and 45.6% (95% CI: 41.0%-50.7%) and 58.4%% (95% CI: 53.6%-63.6%) in LEO. EFS and OS correlated with IPI scores and differed by sPTCL subtypes. ALCL has superior survival, with 2-year EFS and 3-year OS of 79.0% (95% CI: 70.0%-89.0%) and 90.5% (95% CI: 84.0%-97.4%) for ALK+ and 69.4% (95% CI: 60.9%-79.0%) and 79.4% (95% CI: 71.7%-87.9%) for ALK-. Non-ALCL subtypes including PTCL-NOS and AITL have inferior outcomes, with 2-year EFS and 3-year OS of 38.9% (95% CI: 33.3%-45.4%) and 47.3% (95% CI: 41.4%-54.0%) for PTCL-NOS, and 36.2% (95% CI: 29.3%-44.7%) and 57.3% for AITL (95% CI: 49.8%-65.9%).
Conclusion The MER-LEO study is the largest prospective cohort study to date for PTCL in the contemporary era and continues to mature with longitudinal follow-up and ongoing accrual. In comparison to MER, the LEO cohort better reflects the US demographic diversity and begins to incorporate novel agents such as BV in frontline therapy, while most patients in both cohorts continue to receive CHOP-based induction chemotherapy. The overall stability of clinical outcomes within the consecutive MER and LEO cohorts underscores the unmet need of therapeutic breakthrough on a real-world population basis within the past 2 decades, where clinical trials with biomarker-driven targeted approach for non-ALCL frontline treatment are in great need (ClinicalTrials.gov NCT02736357).
Disclosures
Ruan:Pharmacyclics: Research Funding; Kite Pharma: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Seattle Genetics: Consultancy; AstraZeneca: Research Funding. Allen:Kyowa: Consultancy, Honoraria; Daiichi Sanyko: Consultancy, Honoraria. Cohen:Aptitude Health: Consultancy; Lilly Oncology/Eli Lilly: Consultancy, Research Funding; Astrazeneca: Consultancy, Research Funding; Novartis: Research Funding; Takeda: Research Funding; BeiGene: Consultancy, Research Funding; Genentech: Research Funding; HutchMed: Consultancy, Research Funding; Janssen: Consultancy; Kite Pharma/Gilead: Consultancy; BMS/Celgene: Research Funding. Vega:Crisp Therapeutics: Research Funding; Allogene: Research Funding; Geron Corporation: Research Funding. Casulo:Bristol Myers Squibb: Research Funding; Secura Bio: Research Funding; Verastem: Research Funding; Gilead: Research Funding; Genentech: Research Funding. Mehta-Shah:Genetech/Roch: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Corvus Pharmaceuticals: Research Funding; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers-Squibb: Research Funding; Secura Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa Hakko Kirin Co., Ltd.: Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding. Martin:ADCT: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; BMS: Consultancy; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Regeneron: Consultancy; Takeda: Consultancy. Maurer:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Roche/Genentech: Research Funding. Kahl:Beigene: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Pharmacyclics: Consultancy; AcertaPharma: Consultancy; MEI: Consultancy; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Roche: Consultancy; ADT Therapeutics: Consultancy; AstraZeneca: Consultancy, Research Funding; Kite: Consultancy; Janssen: Consultancy; Incyte: Consultancy; Hutchmed: Consultancy, Research Funding; TG Therapeutics: Consultancy; Genmab: Consultancy; Seattle Genetics: Consultancy; Research To Practice: Speakers Bureau. Lossos:Adaptive: Honoraria; NCI: Research Funding; LRF: Membership on an entity's Board of Directors or advisory committees. Flowers:Gilead: Consultancy, Research Funding; Genmab: Consultancy; Guardant: Research Funding; Xencor: Research Funding; EMD: Research Funding; Celgene: Consultancy, Research Funding; Sanofi: Research Funding; Takeda: Research Funding; Morphosys: Research Funding; Kite: Research Funding; TG Therapeutics: Research Funding; SeaGen: Consultancy; Acerta: Research Funding; Spectrum: Consultancy; 4D: Research Funding; NPower: Current holder of stock options in a privately-held company; Adaptimmune: Research Funding; Janssen Pharmaceutical: Research Funding; Iovance: Research Funding; Cellectis: Research Funding; Amgen: Research Funding; Allogene: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Genentech/Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Karyopharm: Consultancy; Pharmacyclics/Janssen: Consultancy; V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Ziopharm: Research Funding; Burroughs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; BeiGene: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding. Cerhan:BMS/Celgene: Research Funding; Genentech: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; NanoString: Research Funding; Protagonist: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal